Because of the generosity of our wonderful community of devoted friends, WE REACHED OUR GOAL of $300,000. The Tisch
MS Research Center is grateful for the tremendous support the trial has received. However, funding is still critically needed for the
study and our stem cell research program, so please continue to donate and make an impact on our progress in treating MS. Thank you from the bottom of our hearts for making a difference in MS research and joining our mission to eradicate MS.
To make a gift, please go to the Tisch MS Research Center Donation Page.
REPAIR, REGENERATE & RENEW: Cutting-Edge Stem Cell Research at
Tisch MS Research Center of New York
In 2013, Tisch MSRCNY made stem cell science history.
Our
adult stem cell treatment received FDA approval for a Phase I Clinical
Trial that has the potential to repair the damage caused by
MS.
This exciting milestone took over ten years to reach and brings indescribable hope to MS patients and their loved ones all over the world.
REPAIR
Our stem cell therapy is the star of the research program at the Tisch
MS Research Center of New York (Tisch MSRCNY), a 501(c)(3), independent, non-profit
research center dedicated to finding the cause of and cure for multiple sclerosis (MS). The Center
focuses on patient-based research, bringing findings rapidly from the laboratory into clinical application to treat symptoms of MS and to halt or reverse
damage caused by the disease.
![]()
About MS
MS is an autoimmune disease of the central nervous system (CNS) that
attacks the myelin sheath that surrounds axons around nerves and can
cause numbness, pain, vision loss, difficulty walking, and even
paralysis.
- MS affects 2.3 million people worldwide
- Over 400,000 people in the U.S. suffer from MS
- Every week, approximately 200 people are diagnosed with MS
- MS affects young adults in their prime as most are diagnosed between age 20-40
- MS affects twice as many women than men
- The cause of MS is not yet known
- Although there are some treatments that may halt progression, there
is no cure for MS
In 2003, Drs. Saud A. Sadiq, Violaine Harris and their research team began
leading the stem cell science revolution by conducting research on strategies to repair the damage caused by MS.
REGENERATE
In 2007, we started the process of obtaining FDA approval for a
Phase I clinical trial. Finally, in August 2013, we received FDA
approval to move ahead!
- The objective of the Phase I trial is to test safety.
- Data from the Phase I trial will lead to an improved strategy for Phase II
- Positive outcomes of the trial may indicate the use of this therapy in other neurological diseases like
ALS and Parkinson's Disease, cerebral palsy and spinal cord injuries.
![]()
Why Stem Cells? In pre-clincal, non-human experiments of our stem cell therapy,
renewal, repair and regeneration of myelin occurred in the CNS after intrathecal (via spinal fluid, not intravenous) injection
.
From
our preclinical models, we know that stem cells injected into the spinal fluid can have significant benefit in terms of improved neurological symptoms. ![]()
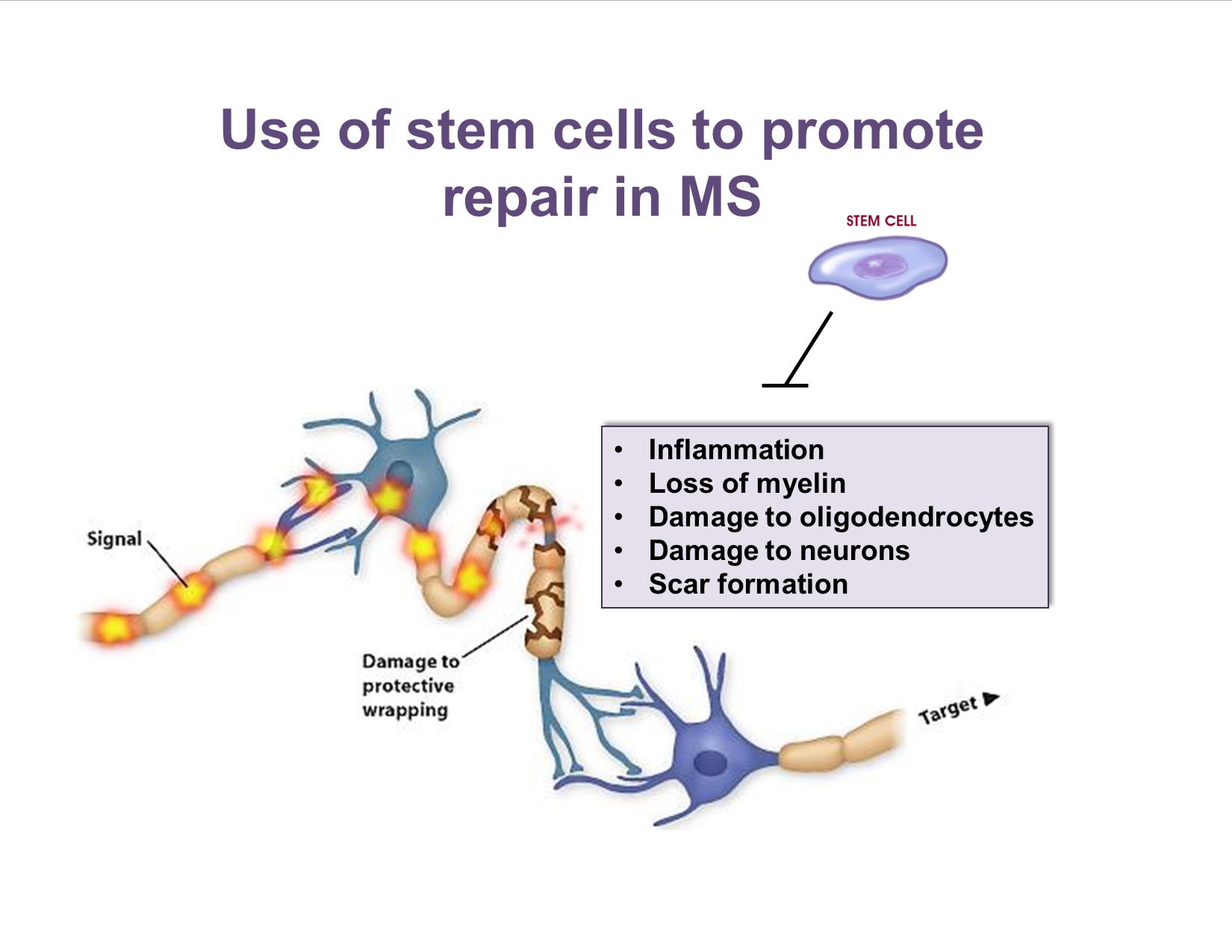
MS is characterized by brain inflammation, loss of myelin, damage to oligodendrocytes (the cells that make myelin), damage to neurons, and scar formation in the brain. As a result, nerve signals are not properly conducted, causing loss of neurological function. Stem cells are hypothesized to promote repair in MS by migrating to areas of demyelination, blocking damage-forming events, and enabling repair.
Why Neural Stem Cells?
Our
unique clinical trial uses adult autologous bone marrow-derived
mesenchymal stem cells as a source for neural progenitors (MSC-NPs) to
promote myelin repair that can potentially reverse or prevent disability in MS patients. Tisch MSRCNY is the only MS Center that has received FDA approval for a stem cell therapy using MSC-NPs for MS patients
.
Procedure![]()
- Bone marrow is taken from patient's hip or sternum
- The sample is taken to the laboratory to isolate and expand the mesenthymal stem cells (MSCs)
- Neural progenitors (NPs) are derived from the MSCs to produce MSC-NPs
- MSC-NPs are injected into the patient's spinal fluid
- This process is repeated once every three months
RENEW
We are eager to start the study.
There's only one thing we now critically need: FUNDING.
We are applying for grants from federal, state and
private entities to help fund the multi-phase study. To get started, we need to equip our lab with essential supplies listed below to conduct the study on the 20 patients.
![]()
![]()
The
cost for each treatment is approximately $10,000, so the total cost for 20 patients who receive 3 treatments each for Phase I is approximately $600,000.
![]()
REASONS TO GIVE
- Tisch MSRCNY has not received federal, state, or corporate funding to cover costs of this study, including supplies, insurance and labor costs
- It is critical to move this clinical trial through the necessary phases of testing in order to validate it as a standard treatment
-
Drive progress in MS research
- Advance the field of regenerative neurology
- Increase knowledge of stem cell science
- Make a direct impact on advancements in MS
-
Bring hope to those who suffer from MS and make a difference in their lives
REACT
Be a part of the stem cell revolution!
-
Share this campaign with Friends on Facebook and Twitter
- Recommend us on Google+
-
Embed this page on your website or blog
-
Email your friends and family to support innovative stem cell
research
Please note: donating to this
campaign will not influence patient recruitment or enrollment in the study.
Read on about our remarkable Center and the stem cell study on the other pages of this campaign!
RESEARCH TEAM
![]()